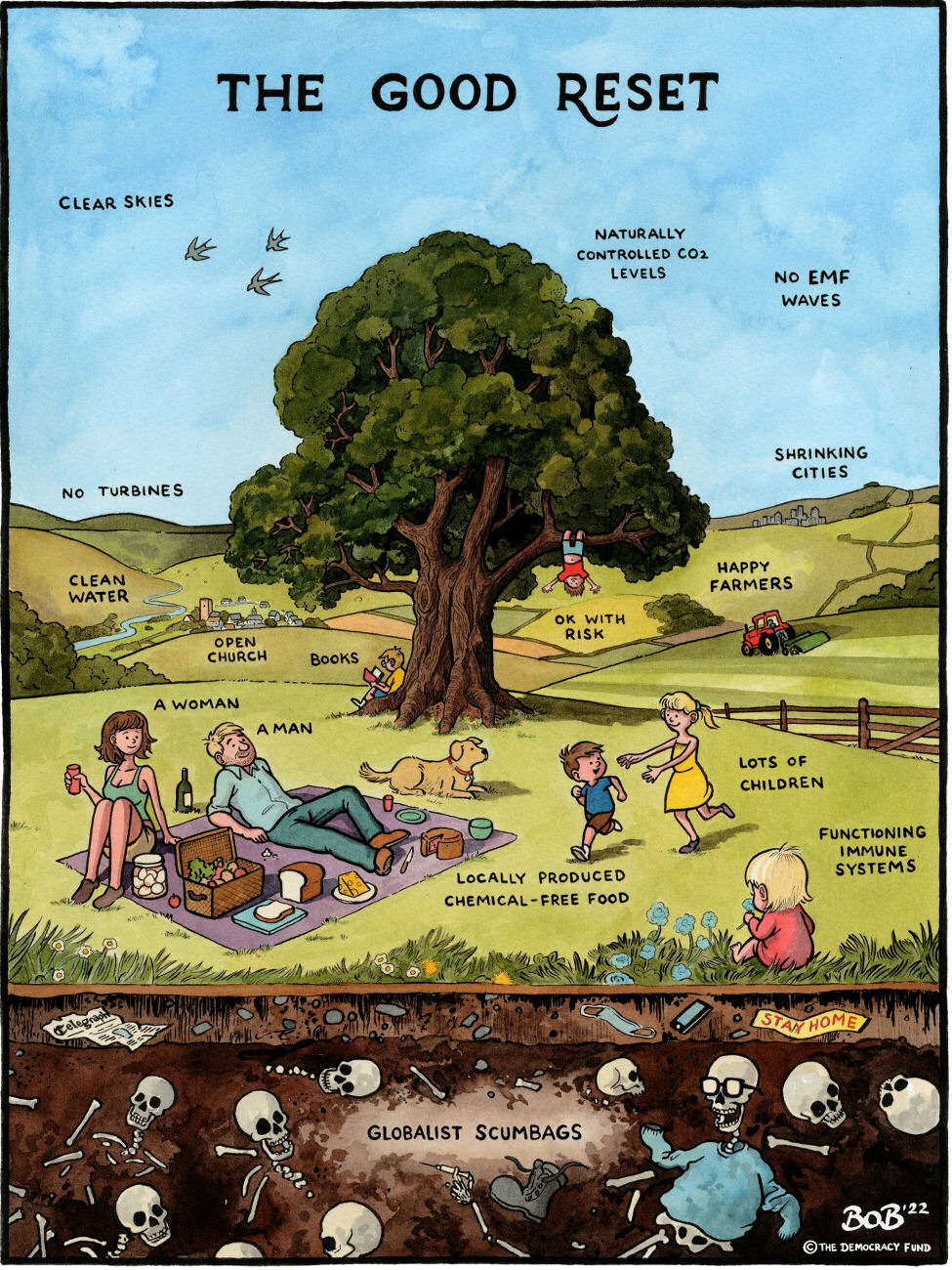
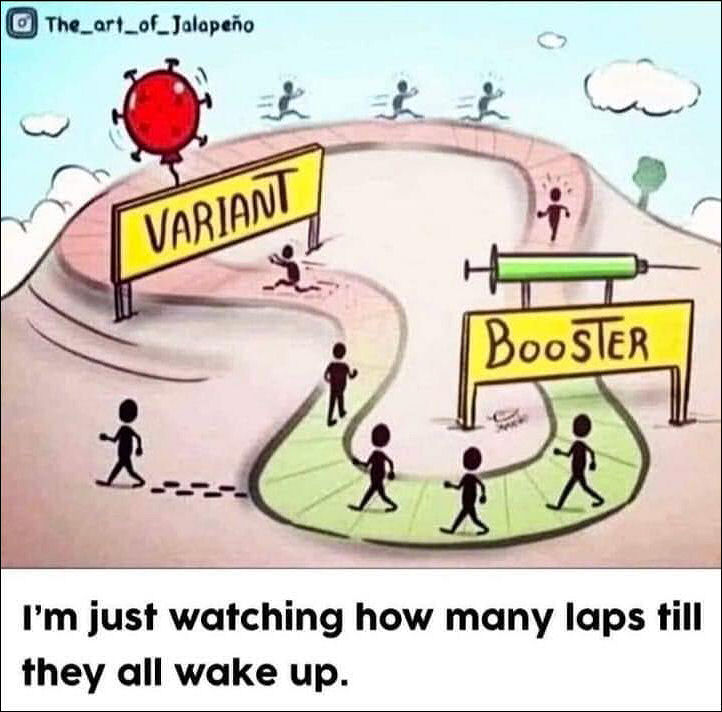
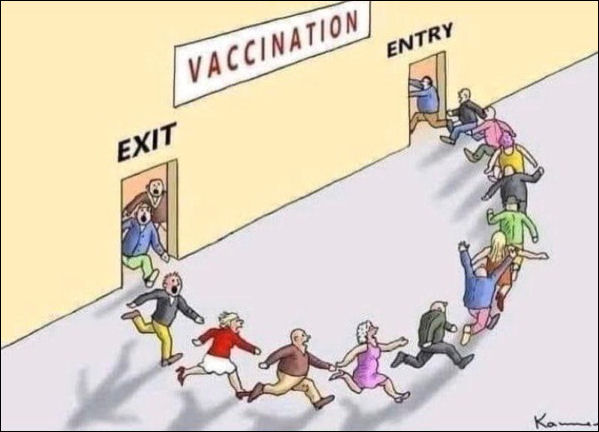
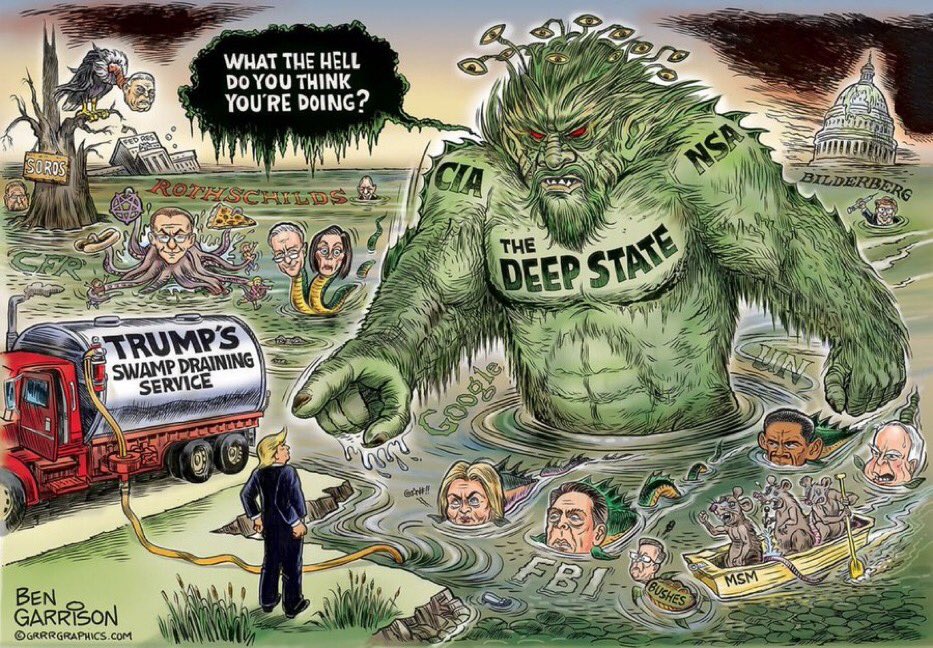
Joshua Rys, Lead Regulatory Scientist for Johnson & Johnson:
"None of that stuff was safe and effective."
"Do you have any idea the lack of research that was done on those products [vaccines]?"
"I mean we basically just had a race to figure out who could solve it best… At one point, we just canned it."
"People wanted it [vaccine], we gave it to them."
"It's like, alright you know what? Cancer patients gonna die anyway."
"I'm sure somebody is going to get sued for that stuff, eventually."
"So like, we didn't do the typical tests."
"The typical process is all this clinical trial testing and stuff in a small population."
"This was just 'let's test it on some lab-rat models, analyze, see if it works and stuff like that and just throw it to the wind and see what happens."
"It was pretty much the government kind of made a deal with phamaceutical companies, and kind of pressured the pharmaceutical companies."
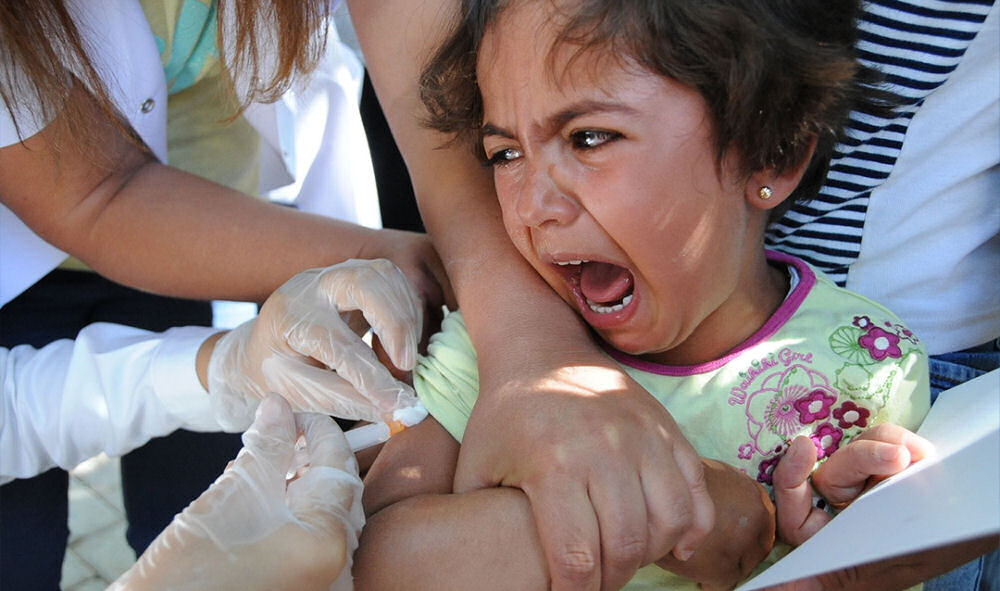
"We didn't do the typical tests," said Joshua Rys, a Lead Scientist in Regulatory Affairs for Johnson & Johnson (J&J), revealed on hidden camera that the typical clinical process was abandoned for the COVID-19 vaccine, knowingly bypassing standard testing protocols under pressure from the U.S. government and public demand. He added, "This was just, 'let's test it on some lab models… and just throw it to the wind and see what happens.'"
He acknowledged that the public wasn't informed about the shortcuts, asking, "Do you have any idea the lack of research that was done on those products?" Rys claimed, "People wanted it, we gave it to them."
While public officials claimed the vaccines were "safe and effective," Rys pushed back. "There's no proof. None of that stuff was safe and effective," he said, adding that the industry relies on a benefit-risk tradeoff to justify product launches.
Rys also pointed to government pressure through Operation Warp Speed. "The government is like, 'We need help… You're solving this problem,'" he said. "People panic, so they try to solve it in whatever way they think is good."
According to a U.S. Department of Health and Human Services (HHS) spokesperson, "Even during a public health emergency, pharmaceutical companies are still required to follow strict protocols for clinical testing. For emergency use, companies must show that the benefits clearly outweigh the risks. Oversight doesn't stop at approval - the FDA and other agencies also monitor products closely once they're in use. That includes real-world safety tracking, independent advisory committees, and required reporting of any adverse events. These steps are in place to make sure public health decisions are based on solid science and strong safeguards - especially in emergencies."
Dr. Marty Makary and Dr. Vinay Prasad recently announced a new vaccine safety and transparency framework - one that's built on gold-standard science, real-world data, and honest communication with the public and will require thorough safety testing before licensing. Their work is focused on strengthening trust, improving how we monitor safety after vaccines are in use, and making sure people have clear, accurate information to make informed decisions.
HHS remains committed to full transparency and evidence-based oversight - putting the safety of the American people first.
OMG has reached out to Joshua Rys and Johnson & Johnson for comment regarding Rys' statements.
Stay tuned for part two. |